
Oxygen is in group 6 of the periodic table. Now, with the Juno spacecraft orbiting Jupiter, oxygen and sulfur ions have been measured above the polar caps with energies up to 400 keV per nucleon (keV/u) (Clark, Mauk, Haggerty, et al., 2017 Clark, Mauk, Paranicas, et al., 2017 Haggerty et al., 2017 ).
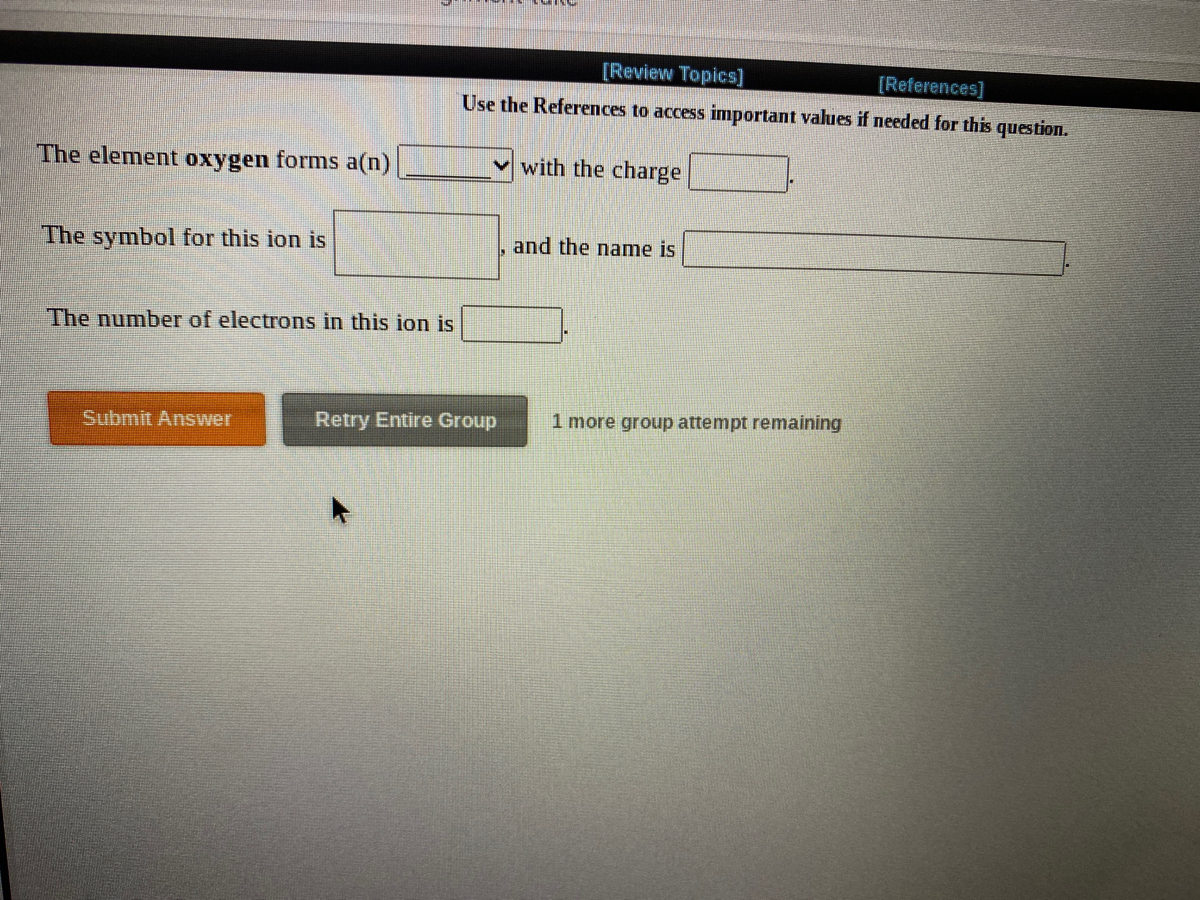
#Oxygen charge of ion plus
So you could write this as platinum with a plus four charge. Four more of the positive thing than you have of the negative things. Sodium is in group 1 of the periodic table. So youre going to have a positive four charge. In this video, we'll walk through this process for the ionic compound calcium bromide. Finally, combine the two ions to form an electrically neutral compound. Then, identify the anion and write down its symbol and charge.
#Oxygen charge of ion full
the ions are positive, because they have more protons than electrons.Loaded 0 Steps of drawing the lewis structure of O 22- There are several steps to draw a lewis structure of a molecule or ion. Usually, we see peroxides as sodium peroxide, potassium peroxide and more. O+2le O2 An electrically-neutral oxygen atom gains two electrons to form an oxygen ion with two negative charges.
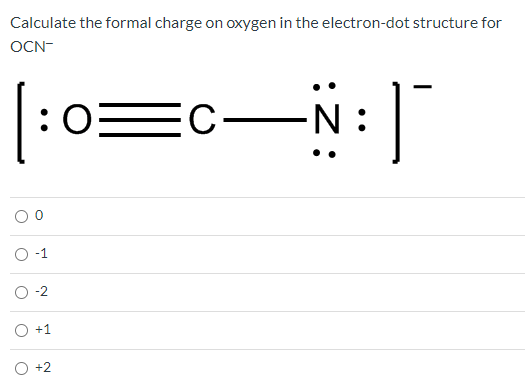
Therefore, overall charge of O 22- is -2. The charge on an ion is equal to the difference in the number of electrons and that of protons it contains- in other words, the number of electrons its parent atom has gained or lost. When oxygen bonds we have found it to either have a formal charge of 0 (2 bonds and 2 lone pairs), +1 (3 bonds and 1 lone pair), and -1 (1 bond and 3 lone pairs). Metal atoms lose electrons from their outer shell when they form ions: Both oxygen atom has -1 charges and three lone pairs. non-metal atoms gain electrons to form negatively charged ions Atomic radii are often measured in angstroms (Å), a non-SI unit: 1 Å 1 × 1010 m 100 pm.metal atoms lose electrons to form positively charged ions.Ions form when atoms lose or gain electrons to obtain a full outer shell: An ion is an atom or group of atoms with a positive or negative charge.


 0 kommentar(er)
0 kommentar(er)
